Introduction: Sickle cell disease (SCD) affects around 100,000 people in the United States, causing acute vaso-occlusive events and chronic end-organ damage. Hydroxyurea (HU) is a proven treatment for SCD that reduces acute and chronic complications. Despite extensive evidence and guideline recommendations, many people with SCD do not receive optimal HU therapy. Our group has shown that using the InCharge Health app, a SCD-specific app co-developed with patients (Alberts et al, JMIR mHealth uHealth, 2020), for at least 24 weeks, increases adherence to HU up to 29%, but engagement with the app varied among users. This study reports user's perceptions of the app relative to their degree of app utilization, thus informing future app adaptations to optimize app engagement and potentiate its effect.
Methods: The InCharge Health app features included daily reminders, adherence and pain progress tracking, communication with providers and other patients, hospital mode deactivation, educational resources, and an accountability partner. Participants used the InCharge Health app and completed the validated Mobile Application Rating Scale (MARS, Stoyanov SR, JMIR mHealth uHealth, 2015) after 24 weeks of app use. The MARS evaluates the quality of mobile health apps by eliciting users' perceptions of engagement, functionality, aesthetics, and information quality. App utilization data (% of the study days) were obtained from the backend of the app and defined as low (<25%), medium low (25-50%), medium high (50-75%), and high (>75%). HU percentage of days covered (PDC), the percentage of days covered by filled HU prescriptions during the study period, was used as a proxy for medication adherence. Baseline pain frequency 12 months before study enrollment was obtained using the Adult Sickle Cell Quality of Life Measurement Information System. After 24 weeks, the ease of use, importance, and helpfulness of app features were evaluated and stratified based on the level of app usage.
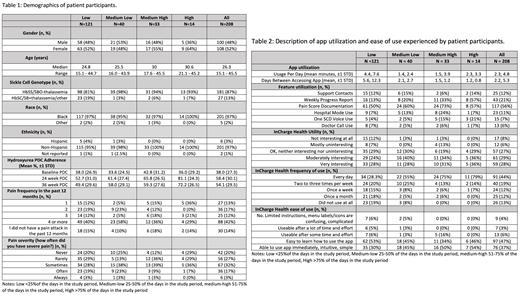
Results: A total of 208 participants enrolled, median age 26.3 years (range, 15 to 45), 52% (108/208) females, 87% (181/208) with HbSS/Sβ 0-thalassemia. Fifty-eight percent (121/208) of participants were low app utilizers, 19.2% (40/208) medium low, 15.9% (33/208) medium high, and 6.7% (14/208) high app utilizers (Table 1). As app usage increased, users reported lower pain frequency and pain severity. After 24 weeks, all groups increased PDC, which sustained post-study, and the magnitude of an increase was proportional to the amount of app use (Table 1). The mean (SD) daily use was 2.3 (4.8) minutes/day, and daily duration of app use increased with the total app use. Conversely, the interval between app usage decreased with total app use (Table 2). The most used app features included pain tracking (56.2%, 117/208) and weekly progress report (20.7%, 43/117). The least used features were connecting to educational resources (7.2%, 15/208) and communicating with treating providers (6.3%, 13/208, Table 2).
Overall, 57.7% (120/208) rated the app moderately or very interesting, 63.0% (131/208) reported using it daily to 2 to 3 days/week, 83.2% (173/208) found the app easy to learn, simple, or intuitive. Low users found the app less interesting and had more concerns of the time and effort it took to use the app (Table 2).
Across all groups, daily reminders were considered the most important and useful feature. Fewer high users considered daily reminders the most important feature, however high users more often deemed other features as most important. HU information was found to be the most important by high users, but this varied between the other user levels. High users also found adherence tracking most important with more users valuing it as app usage increased. Adherence and pain progress tracking were found helpful in all groups except the medium-high users. Importantly, more than 50% of all groups intended to continue using the app beyond the study period.
Conclusions: The InCharge Health app promoted increased HU adherence proportional to its engagement. All users considered daily reminders the most beneficial and crucial feature, while pain tracking was widely utilized. Distinct app consumption and preference patterns were observed according to app usage level. Plans are underway to improve the app functionality and interface to allow real-time customization to boost app engagement and further adherence enhancement among individuals with SCD.
Disclosures
Badawy:Editas Medicine: Consultancy; GBT/Pfizer: Consultancy; Bristol-Myers Squibb: Consultancy; Forma Therapeutics/Novo Nordisk: Consultancy; Vertex Pharmaceuticals Inc: Consultancy; Bluebird bio, INC: Consultancy; Chiesi, Inc: Consultancy. King:GBT: Research Funding; NIH: Research Funding; HRSA: Research Funding; ASH: Research Funding. Gordeuk:Modus Therapeutics: Consultancy; Emmaus: Consultancy, Research Funding; Incyte: Research Funding; Forma: Consultancy, Research Funding; Novartis: Research Funding; GBT/Pfizer: Consultancy, Research Funding; CSL-Behring: Consultancy; Takeda: Consultancy. Shah:Alexion Pharmaceuticals: Speakers Bureau; Agios Pharmaceuticals: Consultancy; Global Blood Therapeutics/Pfizer: Consultancy, Research Funding, Speakers Bureau; Bluebird bio: Consultancy; Vertex: Consultancy; Forma: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal